Enter your Access Code
Adjunct to scaling and root planing to promote attachment level gain and to reduce pocket depth in patients with adult periodontitis.
Collagenase inhibitor drug
DIN: 02247104
Indications
Adjunct to scaling and root planing to promote attachment level gain and to reduce pocket depth in patients with adult periodontitis.
DESCRIPTION
PERIOSTAT® capsule contains doxycycline hyclate which is a semi-synthetic tetracycline. Doxycycline is an inhibitor of collagenase activity. Studies have shown that doxycycline reduces the elevated collagenase activity in the gingival crevicular fluid of patients with chronic adult periodontitis, in an action unrelated to its antibacterial mode of action.
PRESCRIBING INFORMATION
- Periostat® 20 mg twice daily as an adjunct following scaling and root planing may be administered for up to 9 months
- PERIOSTAT® should be taken twice daily
- PERIOSTAT® should be administered at least one hour prior to morning and evening meals
Format
PERIOSTAT® is available as a 20 mg capsule containing doxycycline hyclate for oral administration
Ingredients
Medicinal Ingredient: 20 mg doxycycline hyclate
Non-Medicinal Ingredients: Hard Gelatin Capsules, Magnesium Stearate, Microcrystalline Cellulose and Titanium Dioxide.
Contraindications
This drug is contraindicated in persons who have shown hypersensitivity to doxycycline or any of the other tetracyclines, and in patients with myasthenia gravis. PERIOSTAT® should not be used during tooth development (second half of pregnancy, infancy and in childhood).
Clinical study
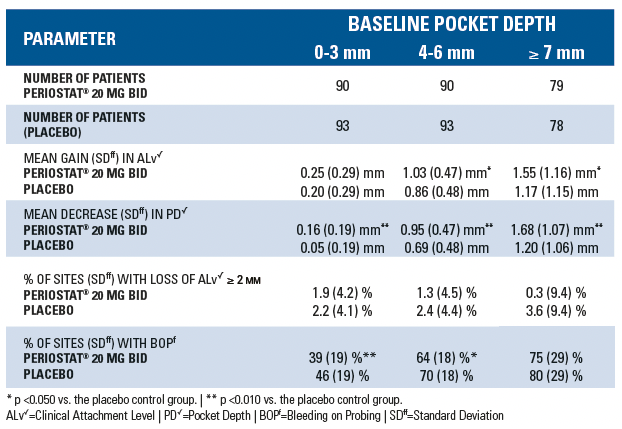
In a randomized, multi-centered, double-blind, 9-month Phase 3 study involving 190 adult patients with periodontal disease [at least two probing sites per quadrant of between 5 and 9 mm pocket depth (PD) and attachment level (ALv)], the effects of oral administration of 20 mg twice a day of doxycycline hyclate plus scaling and root planing (SRP) were compared to placebo control plus SRP. Both treatment groups were administered a course of scaling and root planing in 2 quadrants at Baseline. Measurements of ALv, PD and bleeding-on-probing (BOP) were obtained at Baseline, 3, 6, and 9 months from each site about each tooth in the two quadrants that received SRP using the UNC-15 manual probe. Each tooth site was categorized into one of three strata based on Baseline PD: 0-3 mm (no disease), 4-6 mm (mild/moderate disease), ≥ 7 mm (severe disease). For each stratum and treatment group, the following were calculated at month 3, 6, and 9: mean change in ALv from baseline, mean change in PD from baseline, mean percentage of tooth sites per patient exhibiting attachment loss of ≥ 2 mm from baseline, and percentage of tooth sites with bleeding on probing. The results are summarized in the following table:
ADVERSE REACTIONS
Adverse Reactions in Clinical Trials of PERIOSTAT®In clinical trials of adult patients with periodontal disease 213 patients received PERIOSTAT® 20 mg BID over a 9 - 12-month period. The most frequent adverse reactions occurring in studies involving treatment with PERIOSTAT® or placebo are listed below:
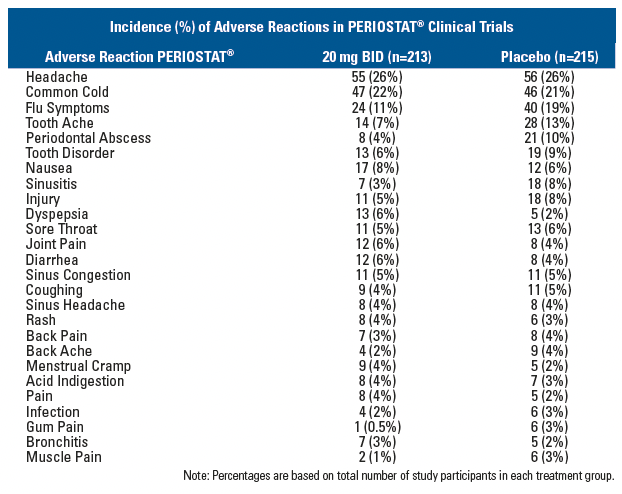 Note: Percentages are based on total number of study participants in each treatment group.
Note: Percentages are based on total number of study participants in each treatment group.
Monograph
Consult the full monograph here
FOR MORE INFORMATIONS
For more informations on PERIOSTAT®, contact Oral Science at 1 888-442-7070 or service@oralscience.com
Adjunct to scaling and root planing to promote attachment level gain and to reduce pocket depth in patients with adult periodontitis
Tetracycline antibiotic
DIN: 02247104
What is PERIOSTAT® used for?
PERIOSTAT® (Doxycycline Hyclate) is used in adults with gum disease (periodontitis) after a certain dental procedure (scaling and root planing). It helps to improve tooth attachment and reduce gum pockets.
PERIOSTAT® contains an antibacterial ingredient called doxycycline that is also used to treat bacterial infections.
How does PERIOSTAT® work?
PERIOSTAT® belongs to the class of antibiotics known as tetracyclines. It may help to prevent the breakdown of gum tissue. PERIOSTAT® capsule contains doxycycline hyclate which is a semi-synthetic tetracycline. Doxycycline is an inhibitor of collagenase activity.
What are the ingredients in PERIOSTAT®?
Medicinal ingredients: Doxycycline hyclate
Non-medicinal ingredients:Hard gelatin capsules, magnesium stearate, microcrystalline cellulose and titanium dioxide.
PERIOSTAT® comes in the following dosage forms:
Capsules - 20 mg
Do not use PERIOSTAT® if you:
- Are allergic or hypersensitive to doxycycline or any other tetracycline antibiotic
- Have the autoimmune disease myasthenia gravis, which causes severe weakness in the muscles used for breathing and moving parts of the body
- Are pregnant or planning to become pregnant. Using PERIOSTAT® during pregnancy can cause birth defects. It can also cause damage and discolouration to your unborn babies developing teeth
- Are breastfeeding or planning to breastfeed. PERIOSTAT® can pass into breastmilk and cause damage and discolouration to your babies developing teeth.
- Prenez des médicaments pour éclaircir le sang, utilisé pour prévenir les caillots sanguins
- You are taking other antibiotics, such as penicillin
- Have a history of oral thrush (yeast infection in your mouth and/or throat) or are at risk for this type of infection.
Other warnings you should know about:
Sun SensitivityPERIOSTAT® can cause your skin to become sensitive to the sun. While taking PERIOSTAT®, use sunscreen and protective clothing if you are going to be in direct sunlight and avoid tanning beds and other sources of UV light. If you notice any skin redness after being in the sun while taking PERIOSTAT® contact your healthcare professional immediately.
Tell your healthcare professional about all the medicines you take, including any drugs, vitamins, minerals, natural supplements or alternative medicines. The following may interact with PERIOSTAT®:- Antacids, used to treat heartburn and indigestion that contain aluminum, calcium, magnesium or bismuth subsalicylate
- Iron-containing preparations, such as iron supplements
- Medicines to thin the blood, used to prevent blood clots
- Antibiotics, used to treat bacterial infections, such as penicillin, tetracycline and methoxyfluorane
- Barbiturates used to treat insomnia and anxiety, such as phenobarbital
- Anti-seizure medicines, such as carbamazepine and phenytoin
- Oral birth control pills
How to take PERIOSTAT®:
- Take PERIOSTAT® twice a day, at least one hour before your morning and evening meals
- PERIOSTAT® capsules should be swallowed with a full glass of water
- Although you may feel better early in treatment, PERIOSTAT® should be used exactly as directed by your healthcare professional
- Misuse or overuse of PERIOSTAT® could lead to the growth of bacteria that will not be killed by doxycycline (resistance). This means that doxycycline will not work for you in the future
- Do not share your medicine.
- 20 mg twice a day for up to 9 months
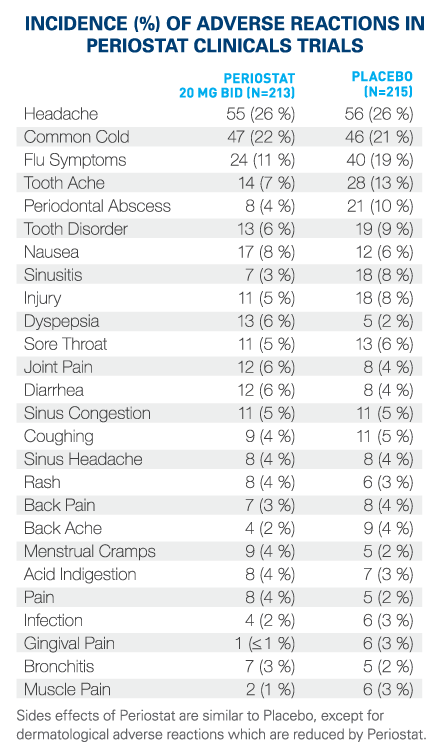
What are possible side effects from using PERIOSTAT®? These are not all the possible side effects you may feel when taking PERIOSTAT®. If you experience any side effects not listed here, contact your healthcare professional.Storage:
Side effects may include:If you have a troublesome symptom or side effect that is not listed here or becomes bad enough to interfere with your daily activities, talk to your healthcare professional.
- Headache
- Common cold (runny nose, sneezing, cough, sore throat)
- Flu symptoms (fever, body aches, headache, cough, sore throat)
- Sinus infection (sinusitis), sinus congestion, sinus headache
- Tooth ache or other tooth problem
- Gum pain
- Nausea, indigestion
- Diarrhea
- Joint pain, back pain/ache, muscle pain
- Rash
- Menstrual cramps.
- Stored at room temperatures of 15°C - 30°C in a tight, light-resistant container
- Protected from excessive humidity
- Keep out of reach and sight of children.
If you want more information about PERIOSTAT®:
- Talk to your healthcare professional
- Find the full product monograph that is prepared for healthcare professionals and includes this Patient Medication Information by visiting the Health Canada website (https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-product-database.html); or by contacting Oral Science at 1-888-442-7070 or service@oralscience.com.

