Nos dernières innovations
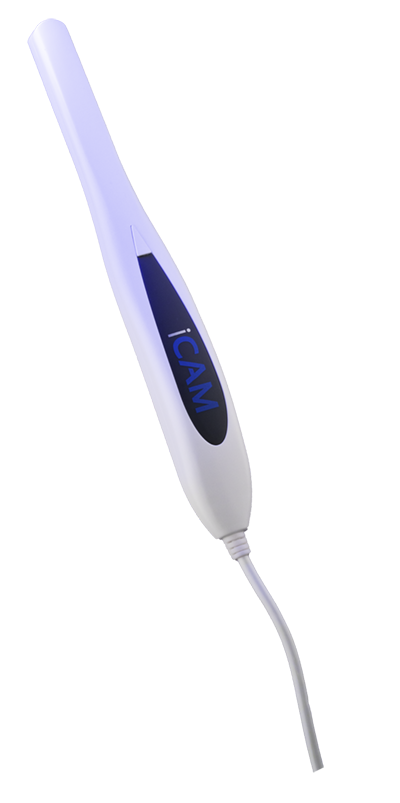

Caméra intra-oral
Faible coût, haute qualité, parfait pour l'hygiène et l'éducation
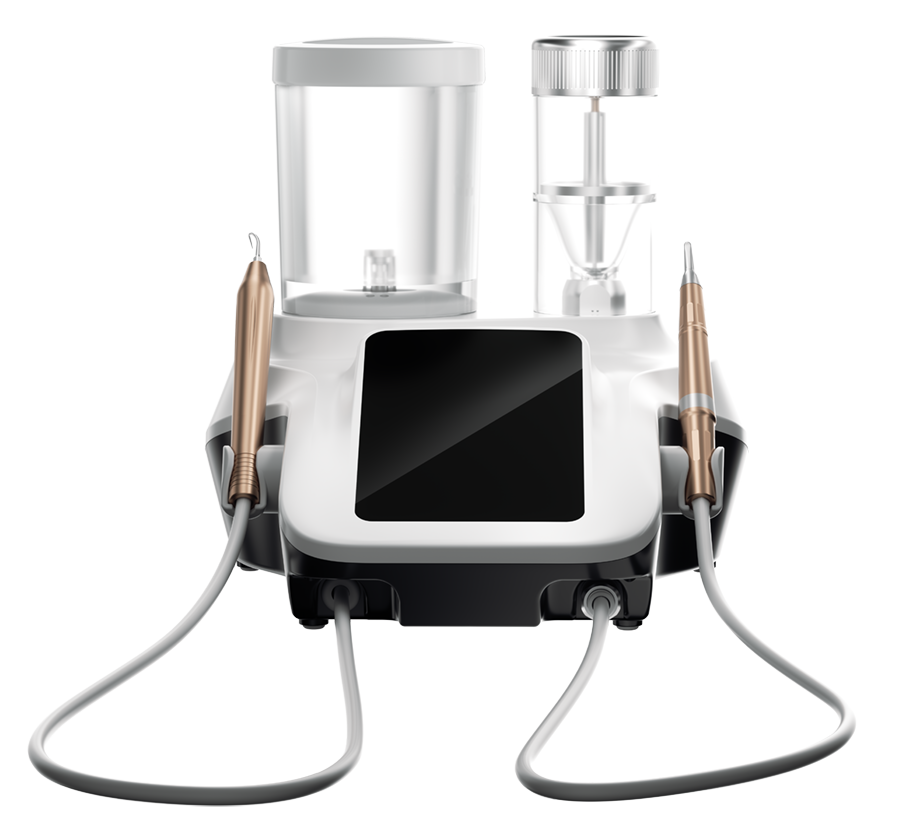

Aéropolisseur et détartreur ultrasonique
Polissage par air, traitement parodontal, traitement endodontique, préparation de la restauration, & maintenance d'implant.


Blanchiment au PAP à la maison
Blanchiment sans douleur avec efficacité supérieure


Désinfectant pour tissus oraux
Accélérez la guérison parodontale avec une technique simple et confortable pour éradiquer tous les micro-organismes dans les poches parodontales
Utilisés par des professionnels dentaires comme vous !